 Beatrice Golinelli-Pimpaneau, UMR 8229 CNRS, College de France, Sorbonne University, Paris
Beatrice Golinelli-Pimpaneau, UMR 8229 CNRS, College de France, Sorbonne University, Paris
I am a research director at the CNRS in the Laboratory of Chemistry of Biological Processes at the Collège de France. I use X-ray crystallography, molecular modeling, site-directed mutagenesis, mass spectrometry and spectroscopy techniques to understand enzyme catalysis at the molecular level.
One of the enzymes I study, RNase Y from Bacillus subtilis acts within the degradosome complex, to initiate messenger RNA degradation in many Gram-positive bacteria. The N-terminal domain of RNase Y (Nter-BsRNaseY), would interact with various protein partners within this complex. Previous analyzes had shown that Nter-BsRNaseY is in equilibrium between two states, monomeric and dimeric, elongated in shape, with a high content of α-helices. Unfortunately, no crystals of this Nter-BsRNaseY domain could be obtained, preventing resolution of its structure by X-ray crystallography.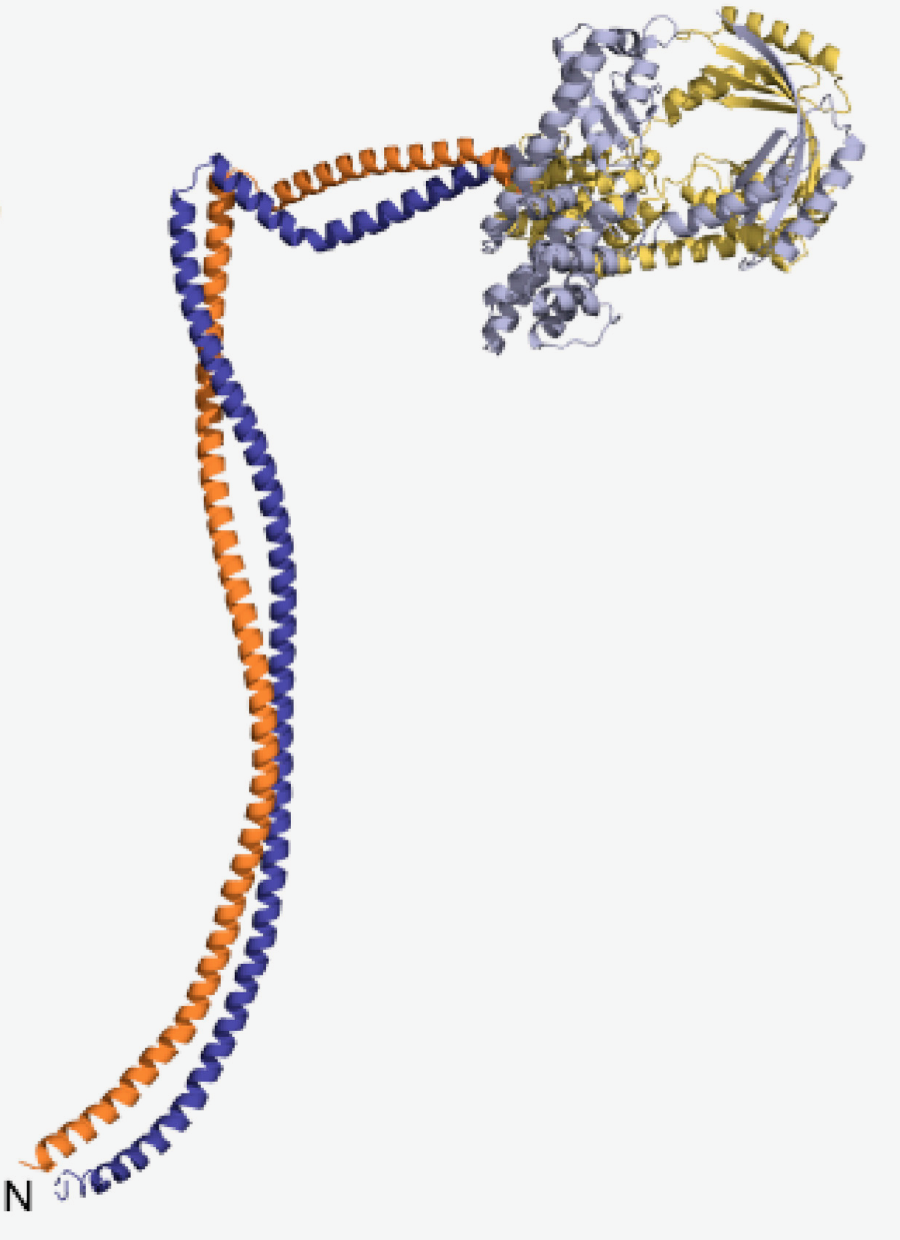
We therefore contacted the NMR team of the ICSN within the framework of Infranalytics. Using multidimensional heteronuclear NMR and three-dimensional AlphaFold predictions, we showed that the Nter-BsRNaseY dimer adopts a coiled-coil structure. Each chain constituting the dimer consists of two long helices linked together by a bend. This structural organization of the Nter-BsRNaseY domain is maintained in the AlphaFold model of the entire RNase Y enzyme. In this model, the globular catalytic domain is made up of two helices linking the KH (RNA-binding domain) and HD (characteristic of the superfamily of metallo-dependent phosphohydrolases) modules, and by the C-terminal region. This one, whose function was still unknown, is very probably involved in the dimerization of RNase Y. This work perfectly illustrates how very high field NMR can answer questions inaccessible by other bio-structural techniques.
More: https://doi.org/10.3390/biom12121798 .
Platform used: ICSN, Gif-sur-Yvette
