Antibiotics disrupt essential mechanisms of bacterial cellular life, which limits bacterial growth (bacteriostatic effect) or kills bacteria (bactericidal effect). However, antibiotics exert natural selection pressure on bacterial populations causing the increasingly frequent emergence of resistant bacteria. This major public health problem is greatly accentuated by the spread of antibiotics and resistant bacteria in the environment. This rapid spread of resistance among bacterial pathogens represents a serious risk to the effectiveness and sustainability of available treatments. In particular, the expression of enzymes called β-lactamases is one of the most important clinical factors for the appearance of resistance in many bacteria, particularly in Gram-negative bacteria, but not only. Under the pressure of antibiotics, the production of β-lactamases by bacteria has effectively become widespread, even by strains that did not previously produce it thanks to the plasmid transfer of genetic information between bacterial cells. All of this β-lactamases, which can be of different types, degrade the antibiotic ring β-lactams, making it inactive. These type antibiotics β-lactam are a large family known to block the activity of PBPs (Penicillin Binding Proteins) and include, among others, penicillin derivatives, cephalosporins, monobactams and carbapenems.
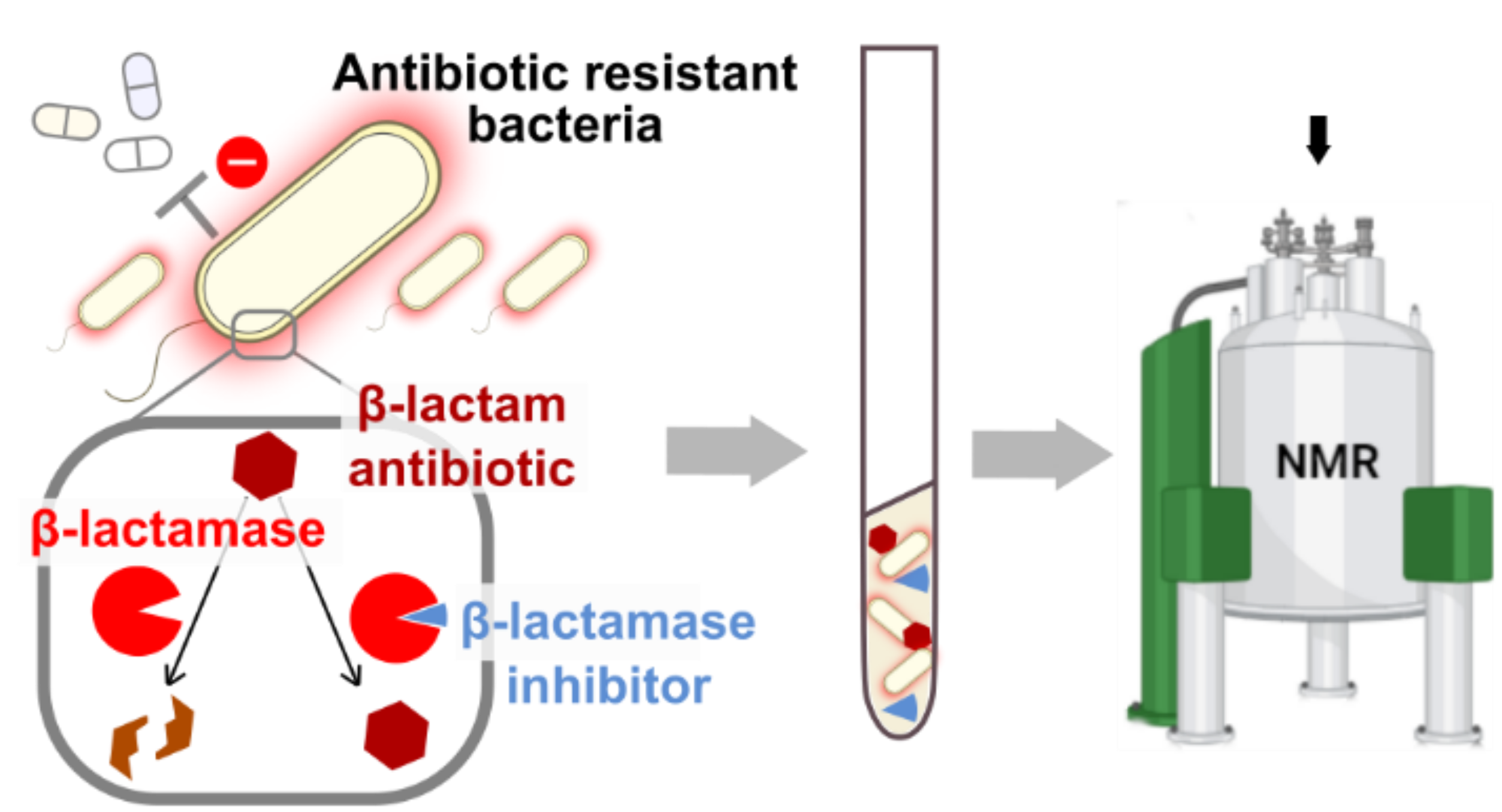
Thanks to NMR applied to samples of whole bacteria, we demonstrate the analytical strength of the method in the in situ and real-time monitoring of (i) the hydrolysis of β-lactams by β-lactamases, (ii) the interaction of drugs belonging to the β-lactam family with their essential targets, the PBPs, and (iii) the binding of inhibitors to these enzymes. In more detail, we optimized the experimental conditions to record the NMR spectra of one of the β-lactamases in situ, carbapenemase KPC-2, which made it possible to monitor the chemical shift disturbances, measured by NMR and caused by the interaction of this β-lactamase with avibactam, the prototypical inhibitor of the family of diazabicyclooctanes. To complement this work, we identified specific peptidoglycan recycling products that accumulate in response to the inhibition of peptidoglycan synthesis enzymes by β-lactam antibiotics and which further act as a molecular signal for induction of β-lactamase in most enterobacteria and in Pseudomonas aeruginosa. Therefore, the ability to monitor peptidoglycan recycling is a way to track the effect of new antibiotics on bacterial physiology and the emergence of resistance. Finally, we demonstrate that our experimental device makes it possible to monitor the activity of β-lactamases on β-lactams and to distinguish this activity from the inactivation of PBPs without selective labeling of β-lactamases or PBPs.
Platform: https://www.ibs.fr/fr/recherche/assemblage-dynamique-et-reactivite/groupe-de-rmn-biomoleculaire/presentation
DOI: 10.1021/jacs.4c00604
