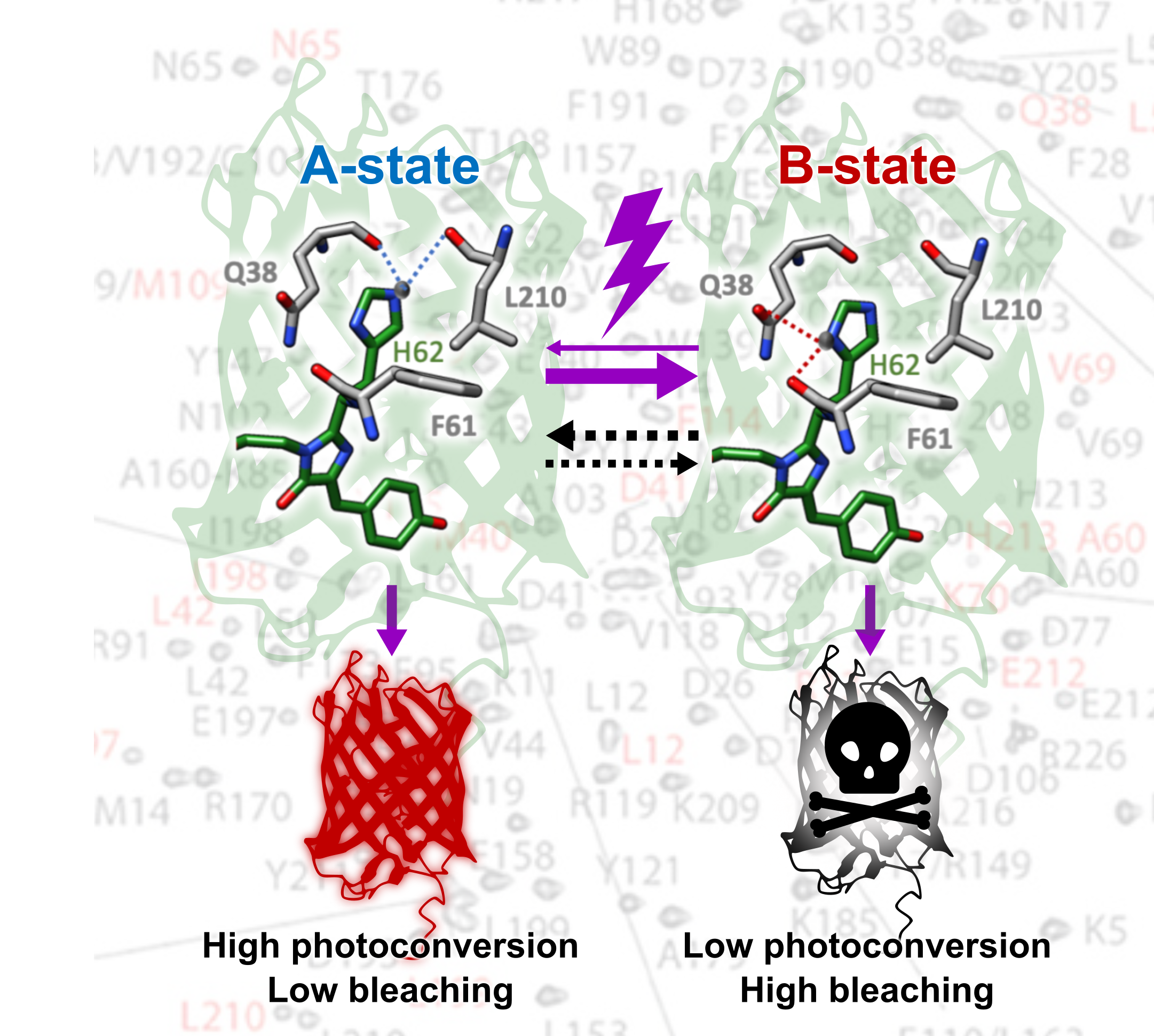
Photoconvertible fluorescent proteins (PCFPs) change their fluorescence emission color from green to red when illuminated by UV light. They are central markers for super-resolution imaging modalities such as quantitative and single-particle tracking single molecule localization microscopy (SMLM). The photophysical properties of these proteins are, however, extremely complex and a new step in this complexity has been revealed by advanced multidimensional NMR spectroscopy combined with fluorescence measurements, using the mEos4b protein. We demonstrated that the mEos4b protein, in its green state, presents two conformations which slowly invert over time. Only one of these conformations photoconverts efficiently to the red state, while the other appears more sensitive to photobleaching. The study helps explain the strange photophysical behaviors, observed experimentally, of mEos4b and related PCFPs. The observed conformational heterogeneity is so subtle that it has never been detected by high-resolution X-ray structures of the protein. Our results reveal how the conformational dynamics of fluorescent proteins can influence their photophysical behavior, help understand the photoconversion mechanism of mEos4b-type PCFPs, and potentially open the door to the design of new variants with higher photoconversion efficiency.
Platform: https://www.ibs.fr/fr/recherche/assemblage-dynamique-et-reactivite/groupe-de-rmn-biomoleculaire/presentation
DOI: 10.1002/advs.202306272
